-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Grants
-
IDF surveys
-
Participating in clinical trials
-
Diagnosing PI
-
Consulting immunologist
-
Clinician education

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Grants
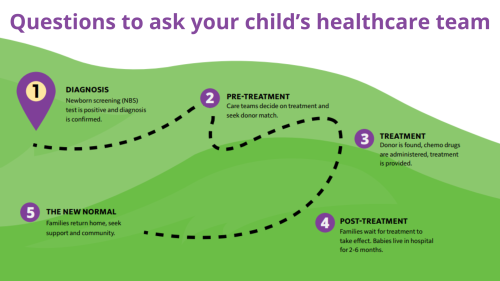
The Food and Drug Administration (FDA) recently approved Orencia for use in the prevention of graft versus host disease (GVHD). The drug was originally created to treat rheumatoid arthritis and has been approved for that use in the U.S. since 2005.
GVHD is a dangerous health condition that can occur after a stem cell transplant, also known as a bone marrow transplant (BMT). In GVHD, the immune system cells from the donor, or “the graft,” attack the person receiving the cells, or “host,” causing a range of symptoms from mild to severe.
Persons with rare primary immunodeficiencies (PI) benefit greatly from BMT. The treatment is common in PIs such as severe combined immune deficiency (SCID), chronic granulomatous disease (CGD), and Wiskott-Aldrich Syndrome (WAS). In many cases, the procedure is lifesaving for these persons.
However, BMT is not without its complications, a major one being GVHD. In GVHD, the new immune system cells, specifically the T cells, from the donor see the existing organs and tissues in the recipient as “foreign.”
The transplant recipient experiences attacks from the donor cells on their skin, and in their gastrointestinal tract, mucous membranes, and certain organs like the liver and lungs. GVHD can be acute or long-term and is potentially fatal. Doctors treat GVHD with steroids and other medications to weaken the new immune system so the donor cells don’t attack the recipient’s body.
Orencia works by preventing T cells from being activated. When T cell activation is interrupted, GVHD is halted.
The drug is potentially useful in adults and children ages 2 and up undergoing BMT from an unrelated donor, and is the first drug approved to prevent GVHD.
“Acute graft versus host disease can affect different parts of the body and become a serious post-transplant complication,” said Dr. Richard Pazdur, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a press release.
“By potentially preventing the disease, more patients may successfully undergo bone marrow or stem cell transplantation with fewer complications.”
Studies conducted on the use of Orencia found that the drug, used in combination with standard immunosuppressive drugs, increased the overall GVHD-free survival rate of persons undergoing BMT from a mismatched unrelated donor.
The FDA’s Orphan Products Grants Program partially supported the studies. The Orphan Products Grants Program provides funding for clinical studies on the safety and efficacy of products for use in rare diseases and conditions.
Related resources
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309