-
Understanding primary immunodeficiency (PI)

Understanding PI
The more you understand about primary immunodeficiency (PI), the better you can live with the disease or support others in your life with PI. Learn more about PI, including the various diagnoses and treatment options.
-
Living with PI
-
Addressing mental health
-
Explaining your diagnosis
- General care
- Get support
- For parents and guardians
-
Managing workplace issues
- Navigating insurance
-
Traveling safely

Living with PI
Living with primary immunodeficiency (PI) can be challenging, but you’re not alone—many people with PI lead full and active lives. With the right support and resources, you can, too.
-
Addressing mental health
-
Get involved

Get involved
Be a hero for those with PI. Change lives by promoting primary immunodeficiency (PI) awareness and taking action in your community through advocacy, donating, volunteering, or fundraising.
-
Advancing research and clinical care
-
Grants
-
IDF surveys
-
Participating in clinical trials
-
Diagnosing PI
-
Consulting immunologist
-
Clinician education

Advancing research and clinical care
Whether you’re a clinician, researcher, or an individual with primary immunodeficiency (PI), IDF has resources to help you advance the field. Get details on surveys, grants, and clinical trials.
-
Grants
By: John G. Boyle, President & CEO
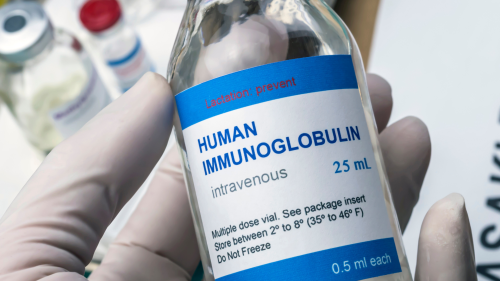
The Immune Deficiency Foundation is focused on providing accurate information to people living with primary immunodeficiency diseases (PI). We regularly hear things—either directly or indirectly—about issues that could affect the availability of immunoglobulin (Ig) products, which are lifesaving, lifelong treatments for people with PI, specifically those who are antibody deficient.
The process of Ig manufacturing
The manufacturing and distribution of Ig is complex because it is a plasma-derived treatment, and the therapy is considered a specialty drug—not something available at your local pharmacy. At any given time there can be a disruption with one or more members of the plasma and Ig supply or delivery chains, including manufacturers, distributors, specialty pharmacies, and various sites of care. Usually these are disruptions that only affect a small number of individuals.
Right now, though, the plasma supply is tight—here in the U.S. and globally. The need for plasma and plasma-derived products grows each year, but the complex nature of Ig products as biologics means that it takes time to increase the supply. It’s not as simple as churning out more pills.
The companies within the plasma and Ig supply and delivery chains make contracts with each other based on historic usage and future projections, and they’re usually accurate enough so shortfalls aren’t experienced regularly. We are currently, however, in a period where every gram of Ig is needed. Therefore, we’re seeing more of these disruptions happen at the same time. Most of those involved hesitate to call these disruptions “shortages” because of their temporary nature and because of how the FDA categorizes shortages. But the main issue is that particular products or vial sizes are temporarily in short supply due to higher usage, not that the Ig market is in peril.
We are in regular communication with the FDA, specifically the Center for Biologics Evaluation and Research (CBER) that regulates biological products for human use. They are keenly aware of the individual product situations and the overall challenges, and they maintain the CBER-Regulated Products: Current Shortages list. Please note that this list is different from the main list that the FDA maintains for drug shortages.
If you’re in a position where you’re being told that the Ig product that you’ve been stabilized on is not available, get ready to ask questions. Between your insurance, your site of care, and a variety of other factors, it may not be easy to get it based on how it’s allocated through distributors or based on what they’re willing to pay—but it’s still out there.
While some people are unable to get the specific Ig product that they prefer, everyone here in the U.S. can ultimately get Ig.
The bottom line is this: the market is tight, but there is product out there.
The shortage solution
So that’s the challenge. The question is: what’s the solution? Unfortunately, there’s not much that can be done in the short term to improve the situation. Neither the FDA, nor Congress, nor the President, nor anyone else can change the playing field at the moment.
IDF is working with those who are seeking to increase yields of Ig from plasma, introduce new fractionation technologies, grow plasma donations at collection centers, and more, but those are long term solutions.
Ultimately, the issue is that the world needs more plasma, and the only good way to make that happen is to collect more plasma. The one thing that we can all do right now is to encourage people to become regular plasma donors if there’s a collection center anywhere near them.
While there is no light at the end of the tunnel when it comes to these disruptions, I have seen more that encourages me rather than discourages me in terms of “increasing the size of the pie” so that no part of the plasma market will ultimately be left without their slice as time goes on.
So if you are faced with someone telling you that your Ig product is not available, question what they’re telling you and get ready to pursue alternative ways of getting what you need, including talking directly with the manufacturer of your product about options that may not have been offered to you.
IDF can’t affect the contracting and distribution challenges that may be behind your particular situation. If you are told that your product is unavailable, please let us know through Ask IDF. That information will help us provide accurate information to others and will help us as we work toward addressing the challenges that all of us who rely on plasma products may face at one point or another.
For information about Ig products, go to www.primaryimmune.org/ig.
This content should not be used as a substitute for professional medical advice. In all cases, patients and caregivers should consult their healthcare providers. Each patient’s condition and treatment is unique. The benefits and risks of any treatment should be discussed with the patient’s provider.
Topics
Sign up for updates from IDF
Receive news and helpful resources to your cell phone or inbox. You can change or cancel your subscription at any time.





The Immune Deficiency Foundation improves the diagnosis, treatment, and quality of life for every person affected by primary immunodeficiency.
We foster a community that is connected, engaged, and empowered through advocacy, education, and research.
Combined Charity Campaign | CFC# 66309